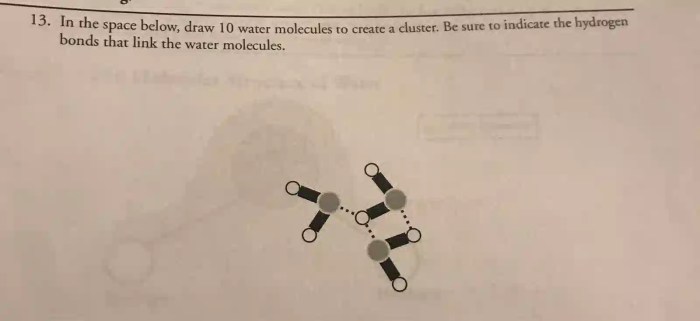
Express your answer as a chemical formula. – In the realm of chemistry, expressing answers as chemical formulas is a fundamental skill that unlocks a deeper understanding of the composition and behavior of substances. This guide delves into the intricacies of chemical formula representation, providing a comprehensive overview of IUPAC guidelines, subscript and superscript usage, and the various types of formulas employed in chemistry.
Chemical Formula Representation
Expressing answers as chemical formulas provides a concise and standardized way to represent the composition and structure of substances. The International Union of Pure and Applied Chemistry (IUPAC) guidelines dictate the format of chemical formulas, ensuring consistency and clarity in scientific communication.
Chemical formulas use symbols to represent elements, and subscripts and superscripts to indicate the number of atoms and ions present. Subscripts are placed after the element symbol to indicate the number of atoms of that element in the molecule or compound.
Superscripts are placed above the element symbol to indicate the charge of an ion.
Types of Chemical Formulas, Express your answer as a chemical formula.
There are three main types of chemical formulas:
- Empirical formulasindicate the simplest whole-number ratio of elements in a compound.
- Molecular formulasrepresent the exact number of atoms of each element in a molecule.
- Structural formulasshow the arrangement of atoms within a molecule, including the bonds between them.
Balancing Chemical Equations

Balancing chemical equations involves adjusting the coefficients in front of the chemical formulas to ensure that the number of atoms of each element is the same on both sides of the equation. This reflects the law of conservation of mass, which states that matter cannot be created or destroyed during a chemical reaction.
The steps involved in balancing chemical equations are as follows:
| Step | Action |
|---|---|
| 1 | Identify the unbalanced equation. |
| 2 | Start by balancing the elements that appear in only one molecule on each side. |
| 3 | Continue balancing the remaining elements, one at a time. |
| 4 | Check if the equation is balanced by counting the atoms of each element on both sides. |
Chemical Reactions: Express Your Answer As A Chemical Formula.
Chemical reactions are processes that involve the transformation of one set of substances into another. There are five main types of chemical reactions:
- Synthesis reactionscombine two or more substances to form a new compound.
- Decomposition reactionsbreak down a compound into two or more simpler substances.
- Single-replacement reactionsinvolve one element replacing another in a compound.
- Double-replacement reactionsinvolve the exchange of ions between two compounds.
- Combustion reactionsinvolve the reaction of a substance with oxygen, releasing energy in the form of heat and light.
| Reaction Type | General Equation | Example |
|---|---|---|
| Synthesis | A + B → AB | 2Na + Cl2 → 2NaCl |
| Decomposition | AB → A + B | 2H2O → 2H2 + O2 |
| Single-replacement | A + BC → AC + B | Zn + 2HCl → ZnCl2 + H2 |
| Double-replacement | AB + CD → AD + CB | NaCl + AgNO3 → NaNO3 + AgCl |
| Combustion | Fuel + O2 → CO2 + H2O | CH4 + 2O2 → CO2 + 2H2O |
Stoichiometry
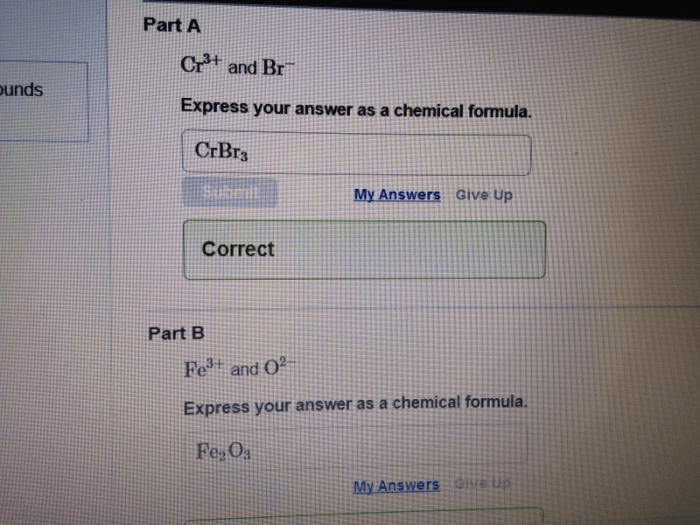
Stoichiometry is the study of the quantitative relationships between reactants and products in chemical reactions. The mole concept is a key tool in stoichiometric calculations, as it provides a way to relate the mass of a substance to the number of particles (atoms, molecules, or ions) present.
The steps involved in stoichiometric calculations are as follows:
| Step | Action |
|---|---|
| 1 | Write a balanced chemical equation for the reaction. |
| 2 | Convert the given mass or volume of one reactant to moles. |
| 3 | Use the stoichiometry of the balanced equation to determine the moles of the other reactants and products. |
| 4 | Convert the moles of the desired substance to mass or volume. |
Question & Answer Hub
What are the benefits of using chemical formulas?
Chemical formulas provide a concise and standardized way to represent the composition of substances, enabling clear communication among chemists and facilitating the understanding of chemical reactions and properties.
How do I determine the empirical formula of a compound?
To determine the empirical formula, you need to know the mass percentages of each element in the compound. Divide the mass percentage of each element by its atomic mass and find the simplest whole-number ratio between these values.
What is the difference between a molecular formula and a structural formula?
A molecular formula represents the exact number and type of atoms in a molecule, while a structural formula provides additional information about the arrangement of atoms within the molecule, including bond connectivity and spatial orientation.