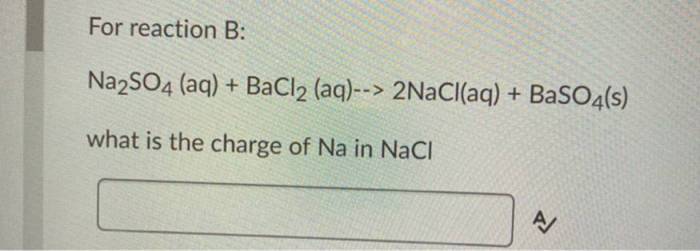Sodium sulfate and barium chloride net ionic equation – The net ionic equation for the reaction between sodium sulfate and barium chloride is a fundamental concept in chemistry that describes the essential chemical change occurring during this reaction. Understanding this equation provides valuable insights into the behavior and properties of these compounds and their interactions.
Sodium sulfate, with the chemical formula Na2SO4, and barium chloride, with the chemical formula BaCl2, are both ionic compounds that dissolve in water to form ions. The net ionic equation focuses on the chemical species that participate directly in the reaction, excluding spectator ions that do not undergo any change.
Sodium Sulfate and Barium Chloride
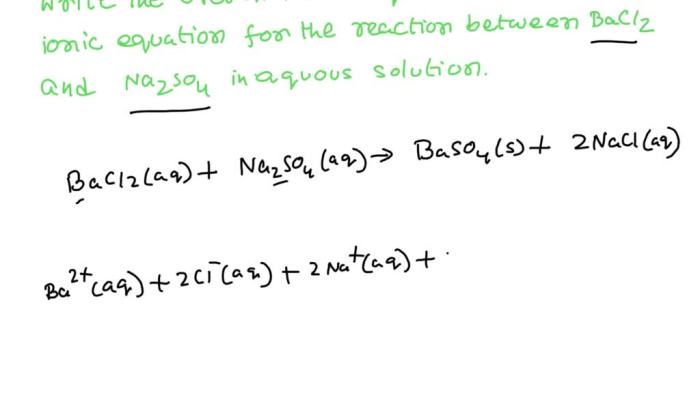
Sodium sulfate (Na 2SO 4) and barium chloride (BaCl 2) are two ionic compounds with distinct properties and applications.
The molar mass of sodium sulfate is 142.04 g/mol, while the molar mass of barium chloride is 208.23 g/mol. Both compounds are soluble in water, but sodium sulfate has a higher solubility than barium chloride.
Net Ionic Equation
A net ionic equation is a chemical equation that shows only the ions that participate in a reaction. To write a net ionic equation, we first need to write the balanced chemical equation for the reaction.
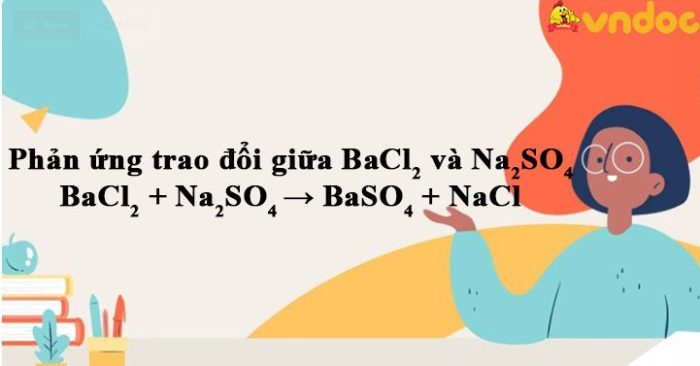
The reaction between sodium sulfate and barium chloride is as follows:
Na 2SO 4(aq) + BaCl 2(aq) → BaSO 4(s) + 2NaCl(aq)
The ions that participate in this reaction are Na +, SO 42-, Ba 2+, and Cl –. The net ionic equation for this reaction is:
Ba 2+(aq) + SO 42-(aq) → BaSO 4(s)
Reaction between Sodium Sulfate and Barium Chloride, Sodium sulfate and barium chloride net ionic equation
The reaction between sodium sulfate and barium chloride is a precipitation reaction. In this type of reaction, one of the products is a solid that precipitates out of solution.
In the reaction between sodium sulfate and barium chloride, the precipitate is barium sulfate (BaSO 4). Barium sulfate is a white, crystalline solid that is insoluble in water.
The other product of the reaction is sodium chloride (NaCl). Sodium chloride is a soluble ionic compound that is commonly known as table salt.
Applications of Sodium Sulfate and Barium Chloride
Sodium sulfate has a variety of industrial uses, including:
- As a filler in paper and textiles
- As a water softener
- As a fertilizer
- As a component of glass and ceramics
Barium chloride has a variety of medical applications, including:
- As a contrast agent in X-ray imaging
- As a treatment for barium poisoning
- As a component of some dental cements
Sodium sulfate and barium chloride are also found in a variety of everyday products, including:
- Sodium sulfate is found in some laundry detergents and cleaning products.
- Barium chloride is found in some fireworks and pyrotechnics.
Essential FAQs: Sodium Sulfate And Barium Chloride Net Ionic Equation
What is the molar mass of sodium sulfate?
142.04 g/mol
What is the solubility of barium chloride in water?
35.7 g/100 mL at 25 °C
What type of reaction occurs between sodium sulfate and barium chloride?
Precipitation reaction