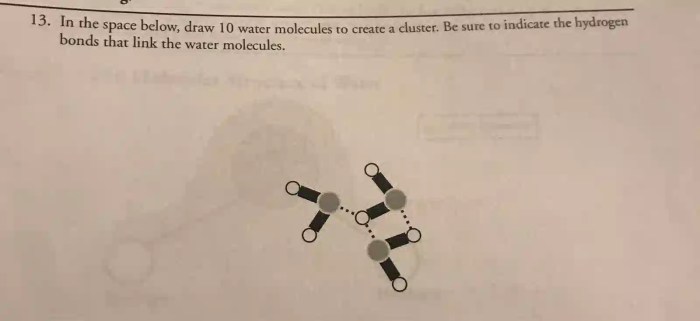
In the realm of chemistry, understanding the behavior of water molecules is paramount. Draw 10 water molecules to create a cluster, a fundamental concept in water chemistry, unveils the intricate interactions and unique properties of these ubiquitous molecules. This exploration delves into the molecular structure, hydrogen bonding, and cluster formation of water, providing a comprehensive overview of their significance in various scientific fields.
Water molecules, composed of two hydrogen atoms covalently bonded to an oxygen atom (H2O), exhibit a unique polarity due to the uneven distribution of electrons. This polarity gives rise to hydrogen bonding, a crucial intermolecular force that governs the behavior of water.
Hydrogen bonding involves the electrostatic attraction between a hydrogen atom covalently bonded to an electronegative atom (such as oxygen) and another electronegative atom. In water, hydrogen bonding occurs between the hydrogen atoms of one molecule and the oxygen atom of another, forming a network of interconnected molecules.
Water Molecule Structure
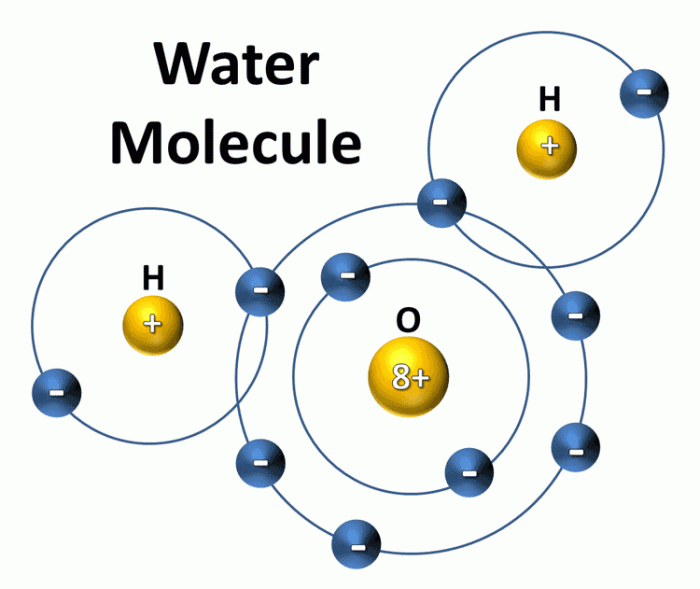
Water molecules consist of two hydrogen atoms covalently bonded to an oxygen atom, forming a bent molecular structure with a bond angle of approximately 104.5 degrees. This molecular geometry results in a polar distribution of electrons, creating a partial positive charge on the hydrogen atoms and a partial negative charge on the oxygen atom.
The polarity of water molecules plays a crucial role in their behavior and interactions with other molecules.
Hydrogen Bonding in Water
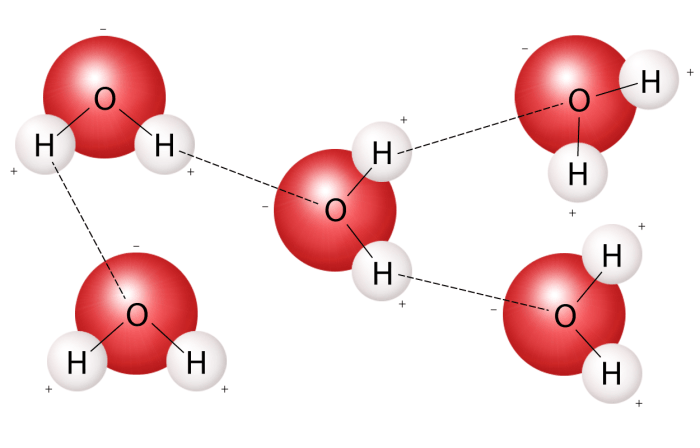
Hydrogen bonding is a type of intermolecular force that occurs between water molecules due to their polarity. The partial positive charge on the hydrogen atoms of one water molecule can interact with the partial negative charge on the oxygen atom of another water molecule, forming a hydrogen bond.
These hydrogen bonds are relatively weak but collectively contribute to the unique properties of water, such as its high surface tension and high specific heat capacity.
Water Cluster Formation
Water clusters are small, dynamic aggregates of water molecules held together by hydrogen bonds. The formation of water clusters is influenced by various factors, including temperature, pressure, and the presence of impurities. As the temperature of water decreases, the tendency for water molecules to form clusters increases.
Types of Water Clusters, Draw 10 water molecules to create a cluster
Water clusters can exist in various sizes and shapes, ranging from small clusters containing a few water molecules to larger clusters consisting of hundreds or even thousands of water molecules. The most common type of water cluster is the tetrahedral cluster, which consists of four water molecules arranged in a tetrahedral geometry.
Other types of water clusters include the hexagonal cluster, the cubic cluster, and the amorphous cluster.
Properties of Water Clusters
Water clusters exhibit unique properties that differ from those of individual water molecules. The size, shape, and stability of water clusters can influence their physical and chemical properties, such as their reactivity, solubility, and transport properties. Water clusters have been found to play a role in various biological processes, including protein folding and enzyme catalysis.
Visualization of Water Clusters
 |
|
Simulation of Water Cluster Dynamics
 |
|
Q&A: Draw 10 Water Molecules To Create A Cluster
What is the significance of hydrogen bonding in water?
Hydrogen bonding is responsible for water’s unique properties, such as its high surface tension, high specific heat capacity, and ability to dissolve many substances.
How do water clusters form?
Water clusters form through hydrogen bonding interactions between water molecules. The size and shape of these clusters depend on various factors, including temperature, pressure, and the presence of solutes.
What are the applications of water clusters?
Water clusters have potential applications in various fields, including drug delivery, nanotechnology, and environmental remediation.